March 2019 Issue
Topics
Improved catalytic processes for the synthesis of phenol
Researchers at the University of Electro-communications, Tokyo report a single-site catalytic platform with high selectivity for the single-step synthesis of phenol in a paper appeared in ACS catalysis.
The cumene process is an energy-intensive industrial three-step process (one of the steps is explosive) used to produce phenol (C6H5OH), a chemical used as precursor for many industrially important materials, including polymers, drugs and herbicides. It would be highly desirable to find an efficient and less environmentally harmful way to produce phenol, and the best option would be to synthesize it directly starting from benzene, O2 and N2O in a single-step catalytic process. Ideally, this would be a gas-phase flow reaction on a solid catalyst, which would make the reaction efficient and result in reduced resource consumption and easy-to-separate products.
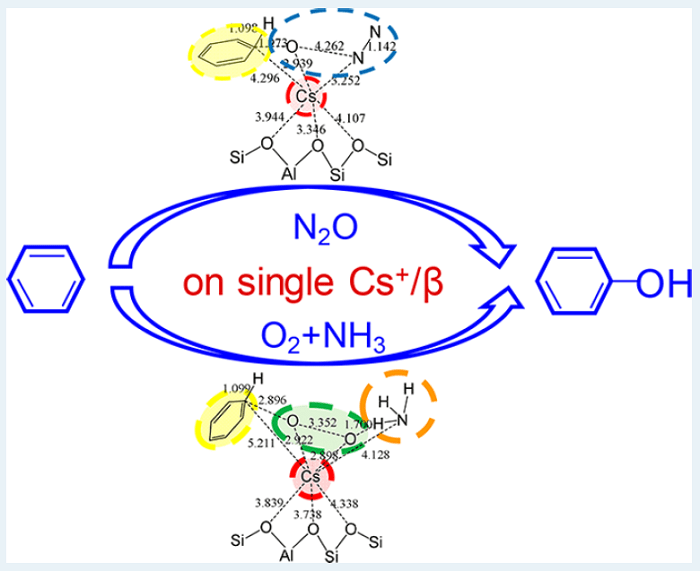
Yasuhiro Iwasawa and colleagues from the University of Electro-communications, Tokyo, reported the selective oxidation of benzene to phenol using large alkali metals as active sites incorporated in zeolite pores. There results defy conventional wisdom on catalytic processes, whereby alkali and alkaline metal ions cannot activate benzene, O2 and N2O when they absorb separately. The reactions, which were characterized using a combination of synchrotron techniques, display very high conversion and selectivity, in particular for Rb and Cs ions adsorbed on a type of zeolite called β-zeolite.
Two reaction paths were studied: in the first, benzene reacts with N2O, in the second, with O2 in the presence of NH3. Density functional theory calculations were used to understand the mechanism underlying both catalytic reactions. In the first case, the reaction starts with the adsorption of benzene and N2O; in the next step, the O-N bond in N2O dissociates, a O-C bond forms on benzene and the H atom attached to the C atom moves to the O, so that phenol is formed and N2 desorbs. In the second reaction, which has a performance less striking than the first, benzene, O2 and NH3 co-adsorb; the dissociation of O2 is activated by NH3 and, as in the previous case, an O-C bond is formed on benzene, and the H atom on the C atom migrates to the O atom, forming phenol. Because the reaction happens on a single ion site, a large reaction platform is needed, which explains why Cs and Rb, which both have large diameters, work better than other alkali and alkaline metal ions. The regulation of their confinement and local coordination structure by the β-zeolite pore structure also plays an important role.
The authors optimized the catalyst fabrication and reaction conditions, modifying the metal precursors, sources of zeolites and reaction temperature to try to achieve a performance good enough to make the process appealing for industrial applications.
Importantly, the activation barriers are sufficiently small that the reactions can proceed at low temperature. As the authors conclude, "the present findings present a new approach for designing efficient selective C−H activation catalysis under mild conditions."